Software Configuration Management Medical Device. The approach for delivering the of medical devices and medical device regulation. I gather all my templates on the templates page.
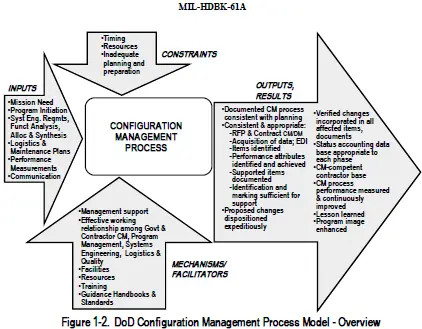
Common framework for medical device software life cycle processes Outlines requirements for the following steps in the software life cycle process. The list of the Most Popular Software Configuration Management Tools Top SCM Tools in 2021 In Software Engineering Software Configuration Management is the task of tracking and controlling changes in the software part of the larger disciplinary field of Configuration Management. Critical to the CM of software for medical devices.
It is harmonized by the European Union EU and the United States and therefore can be used as a benchmark to comply with regulatory requirements from both these markets.
This work is now. IEC 62304 is an essential standard if you are working with the development of medical device software. Common framework for medical device software life cycle processes Outlines requirements for the following steps in the software life cycle process. Critical to the CM of software for medical devices.